
The IMDRF Essential Principles reference dozens of standards from ISO and IEC as well as from other international standards organizations:
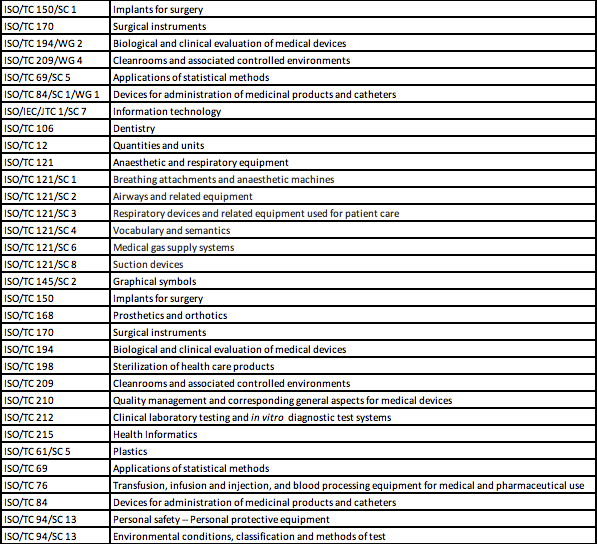
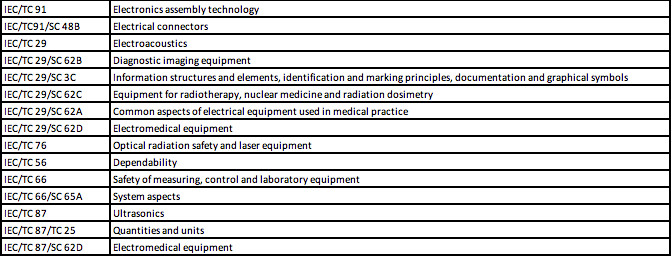
The table below lists the primary ISO and IEC committees responsible for the development of standards that can be used by medical device manufacturers to demonstrate compliance of medical devices with the essential requirements of safety and performance, according to ISO 16142-1 and ISO 16142-2.
ISO Technical Committees and Subcommittees
IEC Technical Committees and Subcommittees
List of ISO/IEC Member Bodies and Participation in Medical Device Committees available in this link.














